Australian Regenerative Medicine company Mesoblast recently suffered a significant financial setback in the ongoing development of MPC-150-IM, their experimental Phase III treatment for chronic heart failure, with their key US

Welsh stem cell company Cell Therapy Ltd. has granted Japanese drugmaker Daiichi Sankyo a license for Heartcel™, its promising cardiac regeneration medicine. Under the conditions of the deal, Daiichi Sankyo
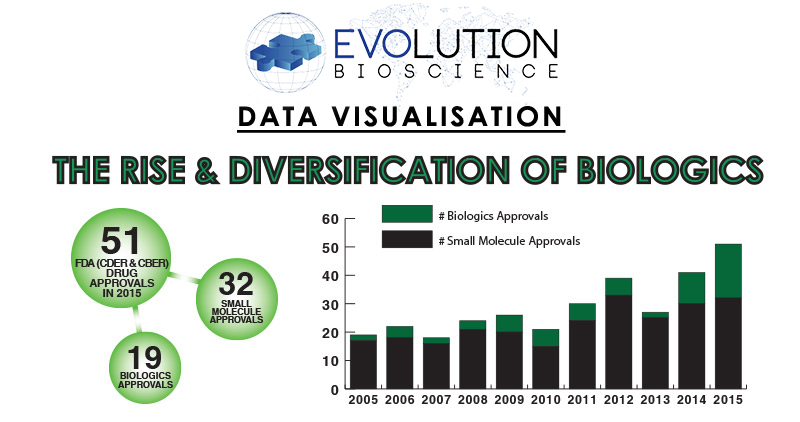
For our latest Data Visualisation project, the Evolution Global Team analysed historical new drug approval statistics and the current clinical trials pipeline to identify trends that highlight the rise and




