As novel types of Biologics have been developed, changes in biomanufacturing approaches have been running in parallel with the intention of maximising yield and lowering production costs. Evolution Bioscience have identified 8 key production trends that will shape the Future of BioManufacturing, the seventh of which is In Silico Modelling.
The Future of Biomanufacturing: In Silico Modelling
INTRODUCTION
In the context of this article, in silico modelling or digital biomanufacturing can be defined as the use of data to model biomanufacturing process options to identify and implement the most optimal scheme possible for scale-up manufacture. The attraction of the in silico modelling approach is based on the significant cost savings achievable by not having to implement several costly smaller scale developments to identify the most appropriate and optimal for progression to larger scale culture.
The goal of in silico modelling is to maximise product yield and productivity by reconstructing and simulating the biomanufacturing process from start to finish. Given the wide remit potential, in silico modelling impacts on all aspects of the biomanufacturing process including:
- Analysis and identification of optimal producer organisms and clone selection strategies
- Development of media and strategies for optimum feeding and culture conditions
- Assessment of the impact of batch and continuous approaches to production
- Development of the most effective purification strategy
UPSTREAM VS. DOWNSTREAM PROCESSING
At its core, in silico modelling focuses on two main parts: upstream processing (USP) and downstream processing (DSP). USP denotes the culturing of the cells in bioreactors and the production of the targeted product. DSP refers to the order of separation/purification techniques used to isolate the product produced via the culture activities. The graphic below outlines key inputs into USP and DSP models:
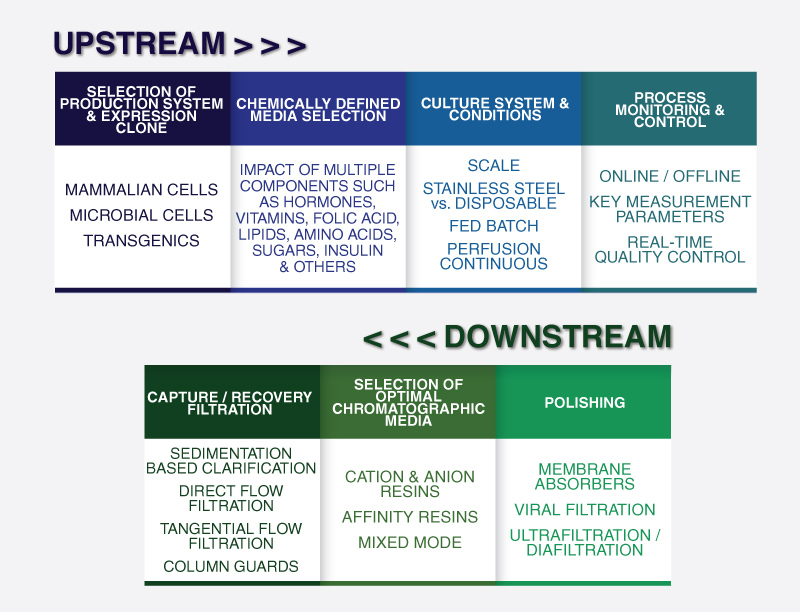
In summary, in silico modelling is concerned with structure, dynamics, control and design principles of a biomanufacturing operation. The process involves the development of mathematical frameworks to simulate the system’s behaviour in response to selection of key manufacturing variables. This facilitates an iterative process of simulation and validation to select the optimal biomanufacturing design prior to physical setup.
MARKET ASSESSMENT: WHO ARE THE KEY PLAYERS?
Whilst the market holds much potential, it is currently only in the early stages of development and is largely driven by academic efforts. Key commercial players offering services to the industry include:
IN SILICO MODELLING: SERVICE PROVISION
| Company | In Silico Offerings |
|---|---|
 | iTope™ and T Cell Epitope Database (TCED™) are in silico tools to rapidly screen antibodies and proteins for potential immunogenicity. |
 | Codexis’ proprietary machine learning software helps determine the additive effect of individual mutations. |
 | Sensitive Immunogenicity Assessment Technology® (SIAT®). SIAT® in silico immunogenicity assessment is based on modern bioinformatics techniques in combination with experimental approaches and can be applied to the prediction of the immunogenicity of biotherapeutic drug candidates including protein, enzyme, antibody, ADC, etc. |
 | Provides ChromX a versatile simulation software application for preparative liquid chromatography processes, that simplifies model-based process development. |
 | Insilico's technology is built around Insilico Cells™, which are genome-based mechanistic network models of common host organisms that are used to produce biologics. These models are refined and calibrated using real-life bioprocess measurements to become an individualized representation of the specific cells in silico. Based on simulations using such models, the behaviour of cells in a bioprocess can be simulated and understood for an almost unlimited number of scenarios. |
 | SuperPro Designer is used throughout the Life Cycle of Product Development and Commercialization to facilitate process optimization, cycle time reduction, improve team collaboration, and shorten the time to market. |
| Lonza’s Epibase® In Silico platform is a patented T cell epitope screening tool for the identification of potential epitopes in biotherapeutic protein and antibody targets. The tool is driven by sequence information, structural bioinformatics, and experimental data. |
Most commercial service developments are focused on epitopic screening to identify the optimal antibody for manufacturing purposes. It should be noted that the industry, as defined by companies developing biotherapeutics, is focused on in silico approaches/applications to chromatographic design and fluid dynamics. This is due the fact that the scale up of both is non-linear.
Table 2 below outlines industry players that are currently developing in silico approaches in-house:
IN SILICO MODELLING: IN-HOUSE/INDUSTRY DEVELOPMENTS
| Company | In Silico Offerings |
|---|---|
 | Developing a highly predictive model for a mAb CEX process. The model uses a novel pH dependent multi-state steric mass action isotherm, calibrated with a limited set of targeted bench scale experiments. The model is validated against a larger set of bench scale experiments. Finally, the model is applied to commercial scale, the results of which can be used for regulatory filing. Amgen are also developing Computational Fluid Dynamics (CFD)-based modelling frameworks for optimizing mixing and aeration-agitation strategies in mixing tanks and bioreactors. |
 | Implementing computational fluid dynamics (CFD) to guide scale up of cell culture bioreactors. |
| Developing mathematical models to identify the fouling mechanism in depth filters for cell culture clarification. | |
 | Developing machine learning approaches for streamlining downstream process development. |
 | Developing mechanistic modelling to study the factors leading to differences in pool sizes observed between scales, and to make predictions on lab scale pool sizes from miniature column data. Also investigating biologics process development enabled via data mining and quantitative structure analysis. |
 | Developing models based on the Donnan equilibrium (DIX) or the Steric Mass Action (SMA) principle were used to predict the elution behaviour of these mAb variants. |
 | Developing process simulations in a clinical scale single-use TFF system examine blend time efficiency. |
 | Developing predictive adsorption models which can reduce screening spaces to specific windows of interest tailored to individual molecules prior to process development. |
 | Using Bayesian techniques, chromatography data from high-throughput and traditional lab-scale DOE studies are combined with manufacturing-scale data to generate models accounting for scaling-offsets. |
 | Developing phase-appropriate in silico models. |
For the concept to expand and provide a holistic system that can facilitate modelling from beginning to end (i.e.: USP through to DSP at scale), access to information such as current process informatics and data historian systems will need to be accessible industry-wide. Such data is the largely the intellectual property of organisations that are known manufacturers of market scale biologics, and as such the sharing of key intellectual property that could simplify the process of competing companies creating biosimilars is unlikely to occur.
INDUSTRY ANALYSIS: CHO-PRODUCED THERAPEUTICS
Given that CHO cells are the predominant cell factory currently in use, the knowledge available and desired to enable in silico developments is largely related to that cell type. Of note is the fact that last year was a record year for this production platform, as highlighted in table 1 below:
CHART 1: ANNUAL CHO-PRODUCED THERAPEUTIC APPROVALS
By analysing the industry players responsible for approved CHO-produced therapeutics, we are able to identify the companies that are likely to own the historical data that would favourably impact the further development of holistic in silico modelling. Genentech/Roche, Lonza, Biogen, Boehringer Ingelheim & Regeneron are the dominant players in this space, as shown in the responsive data visualisation below:
CHART 2: CHO-PRODUCED THERAPEUTICS – INDUSTRY PLAYERS
From a product classification viewpoint the bulk of FDA/EMA approved CHO-produced therapeutics are antibodies, as shown in table 3 below. Given the dominance of antibodies, it is likely that detailed domain knowledge and data in this area will be crucial to progressing in silico techniques.
CHART 3: APPROVED CHO-PRODUCED THERAPEUTICS BY TREATMENT CLASSIFICATION
CONCLUSIONS
Ultimately the question in this field is: can the information exchange required to build highly accurate market scale models be made available in a collaborative industry environment? On one hand such sharing of information will enable much more effective modelling, thereby greatly reducing process development costs. On the other hand, manufacturing of biologics greatly relies upon on internally protected assets that provide a barrier to biosimilar entry post-patent expiration. This presents a catch-22 scenario, and only time will tell which of these opposing and commercially incompatible factors will guide the commercial development of in silico modelling technologies.
Check back soon for Evolution Bioscience’s in-depth analysis of the eighth key production trend in our “Future of BioManufacturing” series, which will focus on Automated Closed Systems for Cell & Gene Therapy Processes.
EVOLUTION BIOSCIENCE WILL HELP YOUR BUSINESS FLOURISH



